
Back حمض التورين Arabic Taurin Azerbaijani تائورین AZB Таўрын Byelorussian Таурин Bulgarian Taurin BS Taurina Catalan Taurin Czech Taurin Danish Taurin German
| |

| |
| Names | |
|---|---|
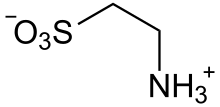
| Preferred IUPAC name
2-Aminoethanesulfonic acid | |
| Other names
Tauric acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.168 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H7NO3S | |
| Molar mass | 125.14 g/mol |
| Appearance | colorless or white solid |
| Density | 1.734 g/cm3 (at −173.15 °C) |
| Melting point | 305.11 °C (581.20 °F; 578.26 K) Decomposes into simple molecules |
| Acidity (pKa) | <0, 9.06 |
| Related compounds | |
Related compounds
|
Sulfamic acid Aminomethanesulfonic acid Homotaurine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Taurine (/ˈtɔːriːn/), or 2-aminoethanesulfonic acid, is a non-proteinogenic naturally occurring amino sulfonic acid that is widely distributed in animal tissues.[1] It is a major constituent of bile and can be found in the large intestine, and accounts for up to 0.1% of total human body weight.
Taurine is named after Latin taurus (cognate to Ancient Greek ταῦρος, taûros) meaning bull or ox, as it was first isolated from ox bile in 1827 by German scientists Friedrich Tiedemann and Leopold Gmelin.[2] It was discovered in human bile in 1846 by Edmund Ronalds.[3]
Although taurine is abundant in human organs with diverse putative roles, it is not an essential human dietary nutrient and is not included among nutrients with a recommended intake level.[4] Taurine is synthesized naturally in the human liver from methionine and cysteine.[5]
Taurine is commonly sold as a dietary supplement, but there is no good clinical evidence that taurine supplements provide any benefit to human health.[6] Taurine is used as a food additive for cats (who require it as an essential nutrient), dogs, and poultry.[7]
Taurine concentrations in land plants are low or undetectable, but up to 1000 nmol/g wet weight have been found in algae.[8][9]
- ^ Schuller-Levis GB, Park E (September 2003). "Taurine: new implications for an old amino acid". FEMS Microbiology Letters. 226 (2): 195–202. doi:10.1016/S0378-1097(03)00611-6. PMID 14553911.
- ^ Tiedemann F, Gmelin L (1827). "Einige neue Bestandtheile der Galle des Ochsen". Annalen der Physik. 85 (2): 326–337. Bibcode:1827AnP....85..326T. doi:10.1002/andp.18270850214.
- ^ Ronalds BF (2019). "Bringing Together Academic and Industrial Chemistry: Edmund Ronald' Contribution". Substantia. 3 (1): 139–152.
- ^ "Daily Value on the New Nutrition and Supplement Facts Labels". US Food and Drug Administration. 25 February 2022. Retrieved 26 August 2023.
- ^ "Taurine". PubChem, US National Library of Medicine. 25 May 2024. Retrieved 31 May 2024.
- ^ "Taurine". Drugs.com. 15 May 2023. Retrieved 26 August 2023.
- ^ EFSA Panel on Additives and Products or Substances used in Animal Feed (2012). "Scientific Opinion on the safety and efficacy of taurine as a feed additive for all animal species". EFSA Journal. 10 (6): 2736. doi:10.2903/j.efsa.2012.2736.
- ^ Kataoka H, Ohnishi N (1986). "Occurrence of Taurine in Plants". Agricultural and Biological Chemistry. 50 (7): 1887–1888. doi:10.1271/bbb1961.50.1887.
- ^ McCusker S, Buff PR, Yu Z, Fascetti AJ (2014). "Amino acid content of selected plant, algae and insect species: a search for alternative protein sources for use in pet foods". Journal of Nutritional Science. 3: e39. doi:10.1017/jns.2014.33. ISSN 2048-6790. PMC 4473169. PMID 26101608.