| |
| Names | |
|---|---|
| Preferred IUPAC name
(E)-Cyclooctene | |
| Other names
trans-Cyclooctene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H14 | |
| Molar mass | 110.200 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.848 g/mL |
| Melting point | −59 °C (−74 °F; 214 K) |
| Boiling point | 143 °C (1 atm); 68-72 °C (100 torr)[2] |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
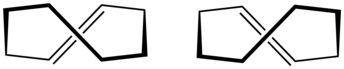
trans-Cyclooctene is a cyclic hydrocarbon with the formula [–(CH2)6CH=CH–], where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor.
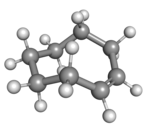
Cyclooctene is notable as the smallest cycloalkene that is readily isolated as its trans-isomer. The cis-isomer is much more stable;[3] the ring-strain energies being 16.7 and 7.4 kcal/mol, respectively.[4]
 |
|
| cis-Cyclooctene in chair conformation |
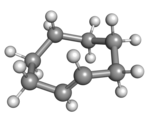
(Rp)-trans-Cyclooctene in crown conformation |
A planar arrangement of the ring carbons would be too strained, and therefore the stable conformations of the trans form have a bent (non-planar) ring. Computations indicate that the most stable "crown" conformation has the carbon atoms alternately above and below the plane of the ring.[5] A "half-chair" conformation, with about 6 kcal/mol higher energy, has carbons 2,3,5,6, and 8 on the same side of the plane of carbons 1,4, and 7.[5]
All conformations of trans-cyclooctene are chiral (specifically, what some call planar-chiral[6]) and the enantiomers can be separated.[7][8][9] In theory, conversion of between the enantiomers can be done, without breaking any bonds, by twisting the whole –CH=CH– group, rigidly, by 180 degrees. However, that entails passing one of its hydrogens through the crowded ring.[7]
- ^ "cis-Cyclooctene". Sigma-Aldrich.
- ^ Cite error: The named reference
vede1973was invoked but never defined (see the help page). - ^ Neuenschwander, Ulrich; Hermans, Ive (2011). "The conformations of cyclooctene: Consequences for epoxidation chemistry". Journal of Organic Chemistry. 76 (24): 10236–10240. doi:10.1021/jo202176j. PMID 22077196.
- ^ Walker, Ron; Conrad, Rosemary M.; Grubbs, Robert H. (2009). "The Living ROMP of trans-Cyclooctene". Macromolecules. 42 (3): 599–605. Bibcode:2009MaMol..42..599W. doi:10.1021/ma801693q. PMC 2850575. PMID 20379393.
- ^ a b Cite error: The named reference
selv2013was invoked but never defined (see the help page). - ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Planar chirality". doi:10.1351/goldbook.P04681
- ^ a b Cope, Arthur C.; Ganellin, C. R.; Johnson, H. W.; Van Auken, T. V.; Winkler, Hans J. S. (1963). "Molecular Asymmetry of Olefins. I. Resolution of trans-Cyclooctene1-3". Journal of the American Chemical Society. 85 (20): 3276–3279. doi:10.1021/ja00903a049.
- ^ Cope, Arthur C.; Mehta, Anil S. (1964). "Molecular Asymmetry of Olefins. II. The Absolute Configuration of trans-Cyclooctene". Journal of the American Chemical Society. 86 (24): 5626–5630. doi:10.1021/ja01078a044.
- ^ Steven D. Paget (2001). "(−)-Dichloro(ethylene)(α-methylbenzylamine)platinum(II)". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rd119. ISBN 0-471-93623-5.
