
Back Transesterifikasie Afrikaans Transesterifikace Czech Umesterung German Transesterificación Spanish Ümberesterdamine Estonian Vaihtoesteröinti Finnish Transesterificación Galician טרנסאסטריפיקציה HE Transesterifikacija Croatian Transesterifikasi ID
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst.[1] Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile. Bases catalyze the reaction by removing a proton from the alcohol, thus making it more nucleophilic. The reaction can also be accomplished with the help of enzymes, particularly lipases (one example is the lipase E.C.3.1.1.3[2]).
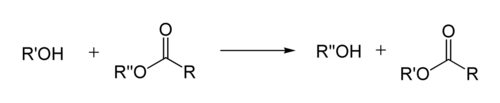
If the alcohol produced by the reaction can be separated from the reactants by distillation this will drive the equilibrium toward the products. This means that esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol.
- ^ Otera, Junzo. (June 1993). "Transesterification". Chemical Reviews. 93 (4): 1449–1470. doi:10.1021/cr00020a004.
- ^ "ENZYME – 3.1.1.3 Triacylglycerol lipase". enzyme.expasy.org. SIB Swiss Institute of Bioinformatics. Retrieved 17 February 2021.