
Back تروبيكاميد Arabic تروپیکامید AZB Tropicamid Welsh Tropicamid German Tropicamida Spanish تروپیکامید Persian Tropikamidi Finnish Tropicamide French Tropikamid Hungarian Տրոպիկամիդ Armenian
 | |
| Clinical data | |
|---|---|
| Trade names | Mydriacyl, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 45% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.673 |
| Chemical and physical data | |
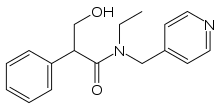
| Formula | C17H20N2O2 |
| Molar mass | 284.359 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tropicamide, sold under the brand name Mydriacyl among others, is a medication used to dilate the pupil and help with examination of the eye.[3] Specifically it is used to help examine the back of the eye.[4] It is applied as eye drops.[3] Effects occur within 40 minutes and last for up to a day.[3]
Common side effects include blurry vision, increased intraocular pressure, and sensitivity to light.[3] Another rare but severe side effect is psychosis, particularly in children.[3] It is unclear if use during pregnancy is safe for the fetus.[5] Tropicamide is in the antimuscarinic part of the anticholinergic family of medications.[3] It works by making the muscles within the eye unable to respond to nerve signals.[3]
Tropicamide was approved for medical use in the United States in 1960.[3] It is on the World Health Organization's List of Essential Medicines.[6]
- ^ "Summary for ARTG Entry: 25356 Mydriacyl tropicamide 0.5% eye drops bottle". Therapeutic Goods Administration. Archived from the original on 5 June 2023. Retrieved 5 June 2023.
- ^ "Mydriacyl 1% eye drops, solution - Summary of Product Characteristics (SmPC)". Electronic Medicines Compendium. 12 February 2020. Archived from the original on 27 June 2022. Retrieved 29 July 2020.
- ^ a b c d e f g h "Tropicamide". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 28 December 2016. Retrieved 8 December 2016.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 314. hdl:10665/44053. ISBN 9789241547659.
- ^ "Tropicamide ophthalmic Use During Pregnancy". Drugs.com. Archived from the original on 28 December 2016. Retrieved 28 December 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.