Back Аӡы Abkhazian Water Afrikaans Wasser ALS ውሃ Amharic Nanum AMI Augua AN Wæter ANG Mun̄ ANN ماء Arabic ܡܝܐ ARC

| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Water | |||
| Systematic IUPAC name
Oxidane (not in common use)[3] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3587155 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.028.902 | ||
| EC Number |
| ||
| 117 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H 2O | |||
| Molar mass | 18.01528(33) g/mol | ||
| Appearance | Almost colorless or white crystalline solid, almost colorless liquid, with a hint of blue, colorless gas[4] | ||
| Odor | Odorless | ||
| Density | |||
| Melting point | 0.00 °C (32.00 °F; 273.15 K) [b] | ||
| Boiling point | 99.98 °C (211.96 °F; 373.13 K)[17][b] | ||
| Solubility | Poorly soluble in haloalkanes, aliphatic and aromatic hydrocarbons, ethers.[8] Improved solubility in carboxylates, alcohols, ketones, amines. Miscible with methanol, ethanol, propanol, isopropanol, acetone, glycerol, 1,4-dioxane, tetrahydrofuran, sulfolane, acetaldehyde, dimethylformamide, dimethoxyethane, dimethyl sulfoxide, acetonitrile. Partially miscible with diethyl ether, methyl ethyl ketone, dichloromethane, ethyl acetate, bromine. | ||
| Vapor pressure | 3.1690 kilopascals or 0.031276 atm at 25 °C[9] | ||
| Acidity (pKa) | 13.995[10][11][a] | ||
| Basicity (pKb) | 13.995 | ||
| Conjugate acid | Hydronium H3O+ (pKa = 0) | ||
| Conjugate base | Hydroxide OH– (pKb = 0) | ||
| Thermal conductivity | 0.6065 W/(m·K)[14] | ||
Refractive index (nD)
|
1.3330 (20 °C)[15] | ||
| Viscosity | 0.890 mPa·s (0.890 cP)[16] | ||
| Structure | |||
| Hexagonal | |||
| C2v | |||
| Bent | |||
| 1.8546 D[18] | |||
| Thermochemistry | |||
Heat capacity (C)
|
75.385 ± 0.05 J/(mol·K)[17] | ||
Std molar
entropy (S⦵298) |
69.95 ± 0.03 J/(mol·K)[17] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−285.83 ± 0.04 kJ/mol[8][17] | ||
Gibbs free energy (ΔfG⦵)
|
−237.24 kJ/mol[8] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Drowning Avalanche (as snow) Water intoxication | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Safety data sheet (SDS) | SDS | ||
| Related compounds | |||
Other anions
|
|||
Related solvents
|
|||
| Supplementary data page | |||
| Water (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
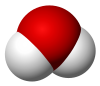
Water is an inorganic compound with the chemical formula H2O. It is a transparent, tasteless, odorless,[c] and nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent[20]). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°.[21] In liquid form, H2O is also called "water" at standard temperature and pressure.
Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas.[22] It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor.
Water covers about 71% of the Earth's surface, with seas and oceans making up most of the water volume (about 96.5%).[23] Small portions of water occur as groundwater (1.7%), in the glaciers and the ice caps of Antarctica and Greenland (1.7%), and in the air as vapor, clouds (consisting of ice and liquid water suspended in air), and precipitation (0.001%).[24][25] Water moves continually through the water cycle of evaporation, transpiration (evapotranspiration), condensation, precipitation, and runoff, usually reaching the sea.
Water plays an important role in the world economy. Approximately 70% of the fresh water used by humans goes to agriculture.[26] Fishing in salt and fresh water bodies has been, and continues to be, a major source of food for many parts of the world, providing 6.5% of global protein.[27] Much of the long-distance trade of commodities (such as oil, natural gas, and manufactured products) is transported by boats through seas, rivers, lakes, and canals. Large quantities of water, ice, and steam are used for cooling and heating in industry and homes. Water is an excellent solvent for a wide variety of substances, both mineral and organic; as such, it is widely used in industrial processes and in cooking and washing. Water, ice, and snow are also central to many sports and other forms of entertainment, such as swimming, pleasure boating, boat racing, surfing, sport fishing, diving, ice skating, snowboarding, and skiing.
- ^ "naming molecular compounds". www.iun.edu. Archived from the original on 24 September 2018. Retrieved 1 October 2018.
Sometimes these compounds have generic or common names (e.g., H2O is "water") and they also have systematic names (e.g., H2O, dihydrogen monoxide).
- ^ "Definition of Hydrol". Merriam-Webster. Archived from the original on 13 August 2017. Retrieved 21 April 2019.
- ^ Leigh, Favre & Metanomski 1998, p. 99.
- ^ Cite error: The named reference
Braun_1993_612was invoked but never defined (see the help page). - ^ a b c Tanaka M, Girard G, Davis R, Peuto A, Bignell N (August 2001). "Recommended table for the density of water between 0 C and 40 C based on recent experimental reports". Metrologia. 38 (4): 301–309. doi:10.1088/0026-1394/38/4/3.
- ^ Lemmon EW, Bell IH, Huber ML, McLinden MO. "Thermophysical Properties of Fluid Systems". In Linstrom P, Mallard W (eds.). NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology. doi:10.18434/T4D303. Archived from the original on 23 October 2023. Retrieved 17 October 2023.
- ^ Lide 2003, Properties of Ice and Supercooled Water in Section 6.
- ^ a b c Anatolievich KR. "Properties of substance: water". Archived from the original on 2 June 2014. Retrieved 1 June 2014.
- ^ Lide 2003, Vapor Pressure of Water From 0 to 370 °C in Sec. 6.
- ^ Lide 2003, Chapter 8: Dissociation Constants of Inorganic Acids and Bases.
- ^ Weingärtner et al. 2016, p. 13.
- ^ "What is the pKa of Water". University of California, Davis. 9 August 2015. Archived from the original on 14 February 2016. Retrieved 9 April 2016.
- ^ Silverstein TP, Heller ST (17 April 2017). "pKa Values in the Undergraduate Curriculum: What Is the Real pKa of Water?". Journal of Chemical Education. 94 (6): 690–695. Bibcode:2017JChEd..94..690S. doi:10.1021/acs.jchemed.6b00623.
- ^ Ramires ML, Castro CA, Nagasaka Y, Nagashima A, Assael MJ, Wakeham WA (1 May 1995). "Standard Reference Data for the Thermal Conductivity of Water". Journal of Physical and Chemical Reference Data. 24 (3): 1377–1381. Bibcode:1995JPCRD..24.1377R. doi:10.1063/1.555963. ISSN 0047-2689.
- ^ Lide 2003, 8—Concentrative Properties of Aqueous Solutions: Density, Refractive Index, Freezing Point Depression, and Viscosity.
- ^ Lide 2003, 6.186.
- ^ a b c d Water in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD)
- ^ Lide 2003, 9—Dipole Moments.
- ^ GHS: PubChem 962 Archived 2023-07-28 at the Wayback Machine
- ^ "Water Q&A: Why is water the "universal solvent"?". Water Science School. United States Geological Survey, U.S. Department of the Interior. 20 June 2019. Archived from the original on 6 February 2021. Retrieved 15 January 2021.
- ^ "10.2: Hybrid Orbitals in Water". Chemistry LibreTexts. 18 March 2020. Archived from the original on 30 July 2022. Retrieved 11 April 2021.
- ^ Butler J. "The Earth – Introduction – Weathering". University of Houston. Archived from the original on 30 January 2023. Retrieved 30 January 2023.
Note that the Earth environment is close to the triple point and that water, steam and ice can all exist at the surface.
- ^ "How Much Water is There on Earth?". Water Science School. United States Geological Survey, U.S. Department of the Interior. 13 November 2019. Archived from the original on 9 June 2022. Retrieved 8 June 2022.
- ^ Gleick, P.H., ed. (1993). Water in Crisis: A Guide to the World's Freshwater Resources. Oxford University Press. p. 13, Table 2.1 "Water reserves on the earth". Archived from the original on 8 April 2013.
- ^ Water Vapor in the Climate System Archived 20 March 2007 at the Wayback Machine, Special Report, [AGU], December 1995 (linked 4/2007). Vital Water Archived 20 February 2008 at the Wayback Machine UNEP.
- ^ Baroni, L., Cenci, L., Tettamanti, M., Berati, M. (2007). "Evaluating the environmental impact of various dietary patterns combined with different food production systems". European Journal of Clinical Nutrition. 61 (2): 279–286. doi:10.1038/sj.ejcn.1602522. ISSN 0954-3007. PMID 17035955.
- ^ Troell M, Naylor RL, Metian M, Beveridge M, Tyedmers PH, Folke C, et al. (16 September 2014). "Does aquaculture add resilience to the global food system?". Proceedings of the National Academy of Sciences. 111 (37): 13257–13263. Bibcode:2014PNAS..11113257T. doi:10.1073/pnas.1404067111. ISSN 0027-8424. PMC 4169979. PMID 25136111.
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).