Back Metamfetamien Afrikaans ميثامفيتامين Arabic Metamfetamin Azerbaijani متامفتامین AZB Shabu BCL Мэтамфэтамін BE-X-OLD Метамфетамин Bulgarian মেথামফেটামিন Bengali/Bangla Metamfetamin BS Metamfetamina Catalan
Artikel ini membahas mengenai narkotika, psikotropika, dan zat adiktif lainnya. Informasi mengenai zat dan obat-obatan terlarang hanya dimuat demi kepentingan ilmu pengetahuan. Kepemilikan dan pengedaran narkoba adalah tindakan melanggar hukum di berbagai negara. Baca: penyangkalan umum lihat pula: nasihat untuk orang tua. |
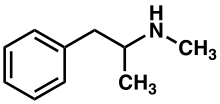
Metamfetamina (metilamfetamina atau desoksiefedrin), disingkat meth, dan dikenal di Asia Tenggara, Hong Kong, Jepang dan Arab Saudi sebagai sabu-sabu atau shabu-shabu,[11][12] adalah obat psikostimulansia dan simpatomimetik. Obat ini dipergunakan untuk kasus parah ADHD atau narkolepsi dengan nama dagang Desoxyn, tetapi juga disalahgunakan sebagai narkotika. "Crystal meth" adalah bentuk kristal dari metamfetamina yang dapat dihisap lewat pipa.
- ^ a b c d e Cruickshank CC, Dyer KR (Juli 2009). "A review of the clinical pharmacology of methamphetamine". Addiction. 104 (7): 1085–99. doi:10.1111/j.1360-0443.2009.02564.x. PMID 19426289.
- ^ a b c d Schep LJ, Slaughter RJ, Beasley DM (Agustus 2010). "The clinical toxicology of metamfetamine". Clinical Toxicology. 48 (7): 675–694. doi:10.3109/15563650.2010.516752. ISSN 1556-3650. PMID 20849327.
- ^ a b Courtney KE, Ray LA (Oktober 2014). "Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature". Drug Alcohol Depend. 143: 11–21. doi:10.1016/j.drugalcdep.2014.08.003. PMC 4164186
 . PMID 25176528.
. PMID 25176528.
- ^ a b Rau T, Ziemniak J, Poulsen D (2015). "The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury". Prog. Neuropsychopharmacol. Biol. Psychiatry. 64: 231–6. doi:10.1016/j.pnpbp.2015.02.013
 . PMID 25724762.
. PMID 25724762. In humans, the oral bioavailability of methamphetamine is approximately 70% but increases to 100% following intravenous (IV) delivery (Ares-Santos et al., 2013).
- ^ "Toxicity". Methamphetamine. PubChem Compound. National Center for Biotechnology Information.
- ^ Sellers EM, Tyndale RF (2000). "Mimicking gene defects to treat drug dependence". Ann. N. Y. Acad. Sci. 909 (1): 233–246. Bibcode:2000NYASA.909..233S. doi:10.1111/j.1749-6632.2000.tb06685.x. PMID 10911933.
Methamphetamine, a central nervous system stimulant drug, is p-hydroxylated by CYP2D6 to less active p-OH-methamphetamine.
- ^ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. hlm. 12–13. Diarsipkan dari versi asli (PDF) tanggal 30 December 2013. Diakses tanggal 30 December 2013.
- ^ Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacol. Ther. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602
 . PMID 15922018.
. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO Diarsipkan 16 September 2018 di Wayback Machine. - ^ Cashman JR, Xiong YN, Xu L, Janowsky A (Maret 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". J. Pharmacol. Exp. Ther. 288 (3): 1251–1260. PMID 10027866.
- ^ a b "Chemical and Physical Properties". Methamphetamine. PubChem Compound. National Center for Biotechnology Information.
- ^ Harsanto, Damar. "Some women inmates unable to kick drug habits". The Jakarta Post.
- ^ Yudono, Jodhi. "Upacara "Spiritual" Kaum Penipu". Kompas.com. Kompas Cyber Media.