
Back Aktinium Afrikaans አክቲኒየም Amharic Actinio AN ऐक्टिनियम ANP أكتينيوم Arabic أكتينيوم ARY اكتينيوم ARZ Actiniu AST Aktinium Azerbaijani Aktinium BAN
 | ||||||||||||||||||||||||||||||||||
| Actinium | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ækˈtɪniəm/ | |||||||||||||||||||||||||||||||||
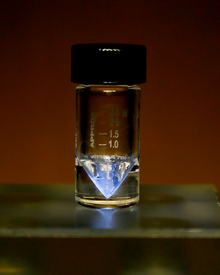
| Appearance | silvery-white, glowing with an eerie blue light;[1] sometimes with a golden cast[2] | |||||||||||||||||||||||||||||||||
| Mass number | [227] | |||||||||||||||||||||||||||||||||
| Actinium in the periodic table | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| Group | f-block groups (no number) | |||||||||||||||||||||||||||||||||
| Period | period 7 | |||||||||||||||||||||||||||||||||
| Block | f-block | |||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 6d1 7s2 | |||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 9, 2 | |||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||
| Melting point | 1500 K (1227 °C, 2240 °F) (estimated)[2] | |||||||||||||||||||||||||||||||||
| Boiling point | 3500±300 K (3200±300 °C, 5800±500 °F) (extrapolated)[2] | |||||||||||||||||||||||||||||||||
| Density (near r.t.) | 10 g/cm3 | |||||||||||||||||||||||||||||||||
| Heat of fusion | 14 kJ/mol | |||||||||||||||||||||||||||||||||
| Heat of vaporization | 400 kJ/mol | |||||||||||||||||||||||||||||||||
| Molar heat capacity | 27.2 J/(mol·K) | |||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||
| Oxidation states | +3 (a strongly basic oxide) | |||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.1 | |||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||
| Covalent radius | 215 pm | |||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||
| Natural occurrence | from decay | |||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) | |||||||||||||||||||||||||||||||||
| Thermal conductivity | 12 W/(m⋅K) | |||||||||||||||||||||||||||||||||
| CAS Number | 7440-34-8 | |||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Friedrich Oskar Giesel (1902) | |||||||||||||||||||||||||||||||||
| Named by | André-Louis Debierne (1899) | |||||||||||||||||||||||||||||||||
| Isotopes of actinium | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
Preview warning: unknown parameter "block"
Preview warning: unknown parameter "isotopes comment"
Preview warning: unknown parameter "isotopes"
Preview warning: unknown parameter "group"
Preview warning: unknown parameter "period"
Actinium is chemical element 89 on the periodic table. Its symbol is Ac. Actinium's mass is 227 g/mol.
Actinium is a silver radioactive, solid metal in actinide group. It is so radioactive that it glows in the dark. Even a small amount of actinium is dangerous to people.
- ↑ Wall, Greg (8 September 2003). "C&EN: It's Elemental: The Periodic Table - Actinium". C&EN: It's Elemental: The Periodic Table. Chemical and Engineering News. Retrieved 2 June 2011.
- ↑ 2.0 2.1 2.2 Kirby, Harold W.; Morss, Lester R. (2006). "Actinium". The Chemistry of the Actinide and Transactinide Elements. p. 18. doi:10.1007/1-4020-3598-5_2. ISBN 978-1-4020-3555-5.
- ↑ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.

