
Back Atoom Afrikaans Atom ALS አቶም Amharic Atomo AN Mot ANG परमाणु ANP ذرة Arabic درة ARY ذره ARZ পৰমাণু Assamese
| Atom | |
|---|---|
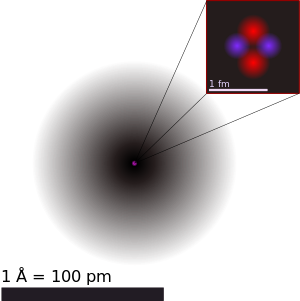 A drawing of the helium atom, showing the nucleus (pink) and the electron cloud (black). The nucleus is made of two protons (red) and two neutrons (violet). The black bar is one angstrom (10−10 m or 0.1 nm). | |
| Properties | |
| Mass range | 1.67×10−27 to 4.52×10−25 kg |
| Diameter range | 62 pm (He) to 520 pm (Cs) (data page) |
| Components | Electrons and a compact nucleus of protons and neutrons |

An atom is an extremely small piece of matter. All normal matter – everything that has mass – is made of atoms. This includes solids, liquids, and gases. The atom cannot be broken to parts by chemistry, so people once thought it was the smallest piece of matter that could exist.[1] There are over 100 different kinds of atoms, called chemical elements. Each kind has the same basic structure, but a different number of parts.
Atoms are very small, but their exact size depends on the type. Atoms are from 0.1 to 0.5 nanometers across.[2] One nanometer is about 100,000 times smaller than the width of a human hair.[3] This makes one atom impossible to see without special tools. Scientists learn how they work by doing experiments.
Atoms are made of three kinds of subatomic particles. These are protons, neutrons, and electrons. Protons and neutrons have much more mass. These are in the middle of the atom, called the nucleus. Lightweight electrons move quickly around them. The electromagnetic force holds the nucleus and electrons together.
Atoms with the same number of protons belong to the same chemical element. Examples of elements are carbon and gold. Atoms with the same number of protons, but different numbers of neutrons, are called isotopes. Usually an atom has the same number of electrons as protons. If an atom has more or less electrons than protons, it is called an ion, and has an electric charge.
Atoms can join by chemical bonds. Many things are made of more than one kind of atom. These are chemical compounds or mixtures. A group of atoms connected by chemical bonds is called a molecule. For example, a water molecule is made of two hydrogen atoms and one oxygen atom. The forming or breaking of bonds is a chemical reaction.
Atoms split if the forces inside are too weak to hold them together. This is what causes radioactivity. Atoms can also join to make larger atoms at very high temperatures, such as inside a star. These changes are studied in nuclear physics. Most atoms on Earth are not radioactive. They are rarely made, destroyed, or changed into another kind of atom.
- ↑ "What is an atom ?". NRC Web. March 19, 2020. Retrieved December 6, 2022.
- ↑ Philip, Michael; Dong, Judy (1998). Elert, Glenn (ed.). "Size of an Atom". The Physics Factbook. Archived from the original on January 30, 2022.
- ↑ Ley, Brian (1999). Elert, Glenn (ed.). "Diameter of a Human Hair". The Physics Factbook. Archived from the original on July 11, 2022.