Back Anilien Afrikaans أنيلين Arabic Anilin Azerbaijani آنیلین AZB Анілін Byelorussian Анилин Bulgarian Anilina Catalan Anilin Czech Anilin Danish Anilin German
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Aniline[1] | |||
| Systematic IUPAC name
Benzenamine | |||
| Other names
Phenylamine
Aminobenzene Benzamine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 605631 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.491 | ||
| EC Number |
| ||
| 2796 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1547 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H5NH2 | |||
| Molar mass | 93.129 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.0297 g/mL | ||
| Melting point | −6.30 °C (20.66 °F; 266.85 K) | ||
| Boiling point | 184.13 °C (363.43 °F; 457.28 K) | ||
| 3.6 g/(100 mL) at 20 °C | |||
| Vapor pressure | 0.6 mmHg (20 °C)[2] | ||
| Acidity (pKa) |
| ||
| −62.95·10−6 cm3/mol | |||
Refractive index (nD)
|
1.58364 | ||
| Viscosity | 3.71 cP (3.71 mPa·s at 25 °C) | ||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
−3394 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
potential occupational carcinogen | ||
| GHS labelling: | |||
    
| |||
| Danger | |||
| H301, H311, H317, H318, H331, H341, H351, H372, H400 | |||
| P201, P202, P260, P261, P264, P270, P271, P272, P273, P280, P281, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P311, P312, P314, P321, P322, P330, P333+P313, P361, P363, P391, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 70 °C (158 °F; 343 K) | ||
| 770 °C (1,420 °F; 1,040 K) | |||
| Explosive limits | 1.3–11%[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LDLo (lowest published)
|
195 mg/kg (dog, oral) 250 mg/kg (rat, oral) 464 mg/kg (mouse, oral) 440 mg/kg (rat, oral) 400 mg/kg (guinea pig, oral)[4] | ||
LC50 (median concentration)
|
175 ppm (mouse, 7 h)[4] | ||
LCLo (lowest published)
|
250 ppm (rat, 4 h) 180 ppm (cat, 8 h)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (19 mg/m3) [skin][2] | ||
REL (Recommended)
|
Ca [potential occupational carcinogen][2] | ||
IDLH (Immediate danger)
|
100 ppm[2] | ||
| Related compounds | |||
Related aromatic amines
|
1-Naphthylamine 2-Naphthylamine | ||
Related compounds
|
Phenylhydrazine Nitrosobenzene Nitrobenzene | ||
| Supplementary data page | |||
| Aniline (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
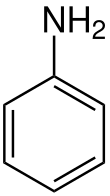
Aniline (from Portuguese anil 'indigo shrub', and -ine indicating a derived substance)[6] is an organic compound with the formula C6H5NH2. Consisting of a phenyl group (−C6H5) attached to an amino group (−NH2), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds.[7] It is toxic to humans.
Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a diazonium salt, which can then undergo various nucleophilic substitution reactions.
Like other amines, aniline is both a base (pKaH = 4.6) and a nucleophile, although less so than structurally similar aliphatic amines.
Because an early source of the benzene from which they are derived was coal tar, aniline dyes are also called coal tar dyes.
- ^ Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 416, 668. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Aniline, for C6H5-NH2, is the only name for a primary amine retained as a preferred IUPAC name for which full substitution is permitted on the ring and the nitrogen atom. It is a Type 2a retained name; for the rules of substitution see P-15.1.8.2. Substitution is limited to substituent groups cited as prefixes in accordance with the seniority of functional groups explicitly expressed or implied in the functional parent compound name. The name benzenamine may be used in general nomenclature.
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0033". National Institute for Occupational Safety and Health (NIOSH).
- ^ Vollhardt, P.; Schore, Neil (2018). Organic Chemistry (8th ed.). W. H. Freeman. p. 1031. ISBN 9781319079451.
- ^ a b c "Aniline". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Aniline". cameochemicals.noaa.gov. US NOAA Office of Response and Restoration. Retrieved 2016-06-16.
- ^ "aniline | Etymology, origin and meaning of aniline by etymonline". www.etymonline.com. Retrieved 2022-02-15.
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page).