
Back Diffusie Afrikaans انتشار Arabic Diffuziya Azerbaijani Дыфузія Byelorussian Дыфузія BE-X-OLD Дифузия Bulgarian ব্যাপন Bengali/Bangla Difuzija BS Difusió Catalan بڵاوبوونەوە CKB
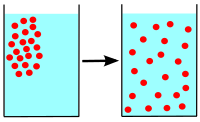
Diffusion is a process where molecules of a material move from an area of high concentration (where there are many molecules) to an area of low concentration (where there are fewer molecules)[1]until it has reached equilibrium (molecules evenly spread).
Diffusion usually happens in a mixture in gas, a liquid and occasionally colloids. It is possible to see diffusion happening when two liquids are mixed in a transparent container. It describes the constant movement of particles in all liquids, gases and colloids. These particles move in all directions bumping into each other.